Section summary #
All samples trashed due to freak evaporation event.
Sample preparations #
3/4/22 #
T7Init Mix 1 BamHI-HF digest #
Digested 4 ug T7Init Mix 1 (12.90 ul) using 4 ul NEB BamHI-HF and 10x rCutSmart buffer in total volume of 150 ul. Made an equivalent un-digested control to use as the supercoiled substrate. Allowed samples to incubate at 37C in the hot room for 30 mins after which I preformed phenol/chloroform extraction using homemade phaselock tubes and EtOH precipitation. Resuspended samples in 40 ul (100 ng/ul) Tris-HCl.
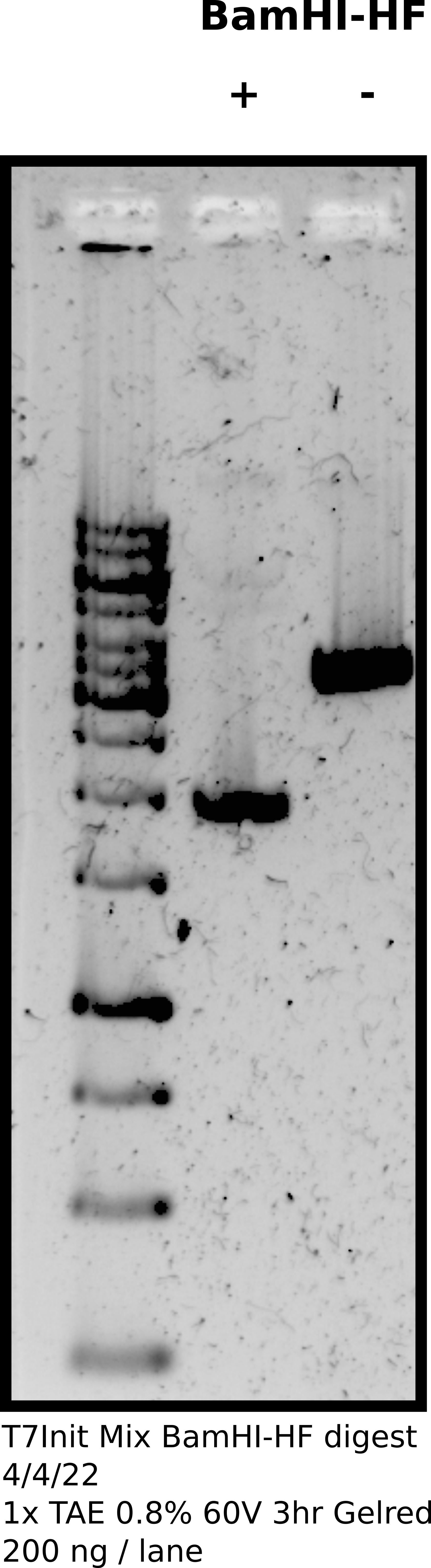
Agarose gel digested samples #
Confirmed digestion on agarose gel.
3/5/22 #
IVT and Bisulfite conversion #
Prepared BamHI digested and supercoiled samples for bisuflite treatment according to this protocol. Sample descriptions are shown in the table below. Complete sample and reagent details can be found in this spreadsheet.
EHS1 sample table
| Sample Series | Sample Name | Sample Number | Formal name | Transcribed | TopoisomeraseI | Gyrase | BamHI-HF | RnaseA | RnaseH | Deprotienzied | ng/ul | 260/280 | 260/230 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHS1 | 0 | EHS1-R0 | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | 11.3 | 1.792 | -2.7 | |
| EHS1 | 1 | EHS1-R1 | TRUE | FALSE | FALSE | FALSE | FALSE | FALSE | FALSE | 45 | 2.248 | -95 | |
| EHS1 | 2 | EHS1-R2 | TRUE | FALSE | FALSE | TRUE | FALSE | FALSE | FALSE | 90.6 | 1.827 | 0.973 | |
| EHS1 | 3 | EHS1-R3 | TRUE | FALSE | FALSE | TRUE | FALSE | FALSE | FALSE | 56.2 | 1.891 | 1.347 |
Only deviation from usual protocol was incubated in the hot room on a rotator as the rotisserie oven was in use.
Big trouble #
I cam back to pick up the proteinized samples after the 2 hr bisulfite incubation and found that one of the samples had a significant of weird goop and almost none of the 150 ul of sample remaining (image of goo shown below).

After talking to Meghan and Fred about both suspected damaged tube which resulted in evaporation. Given that I can’t be sure that other samples did not also undergo some but less dramatic degrees of evaporation I choose to trash all samples.
Next time will put Parafin wrap on lids of tubes to prevent evaporation.
Note on replicates #
Fred suggested will need 2 replicates (separate transcription reactions) for each treatment. For next replicate preparation double everything up to get 2 replicates.