11/29/21 #
VR16 KpnI digest #
Sanger seq results for some of the VR16 mini preps were not the cleanest. Some died in GC rich region. Running KpnI digest gel to determine which samples to sequence again if needed in reverse direction.
Digest setup #
| Reagent | Volume (ul) |
|---|---|
| Plasmid | 4 |
| KpnI | 0.5 |
| 1.1 10x buffer | 1 |
| npH20 | 4.5 |
Digested samples for 1 hour at 37C in thermocycler.
Preparing VR24 fragment #
Received VR24 insert in vector. 5 ug total mass. Re-suspended in 100 ul TE for final concentration 50 ug/ul.
VR24 vector BglII digest #
Digest VR24 vector to free fragment.
Digest conditions #
| Reagent | Volume (ul) |
|---|---|
| VR24 plasmid | 0.5 (25 ng) |
| BglII | 0.5 |
| 3.1 10x buffer | 2 |
| npH20 | 17 |
Vector was digested for 1 hour at 37C in the thermocycler.
PCR amplification of VR24 BglII digest product #
PCR master mix preparation #
Prepared PCR master mix in the tissue culture hood using the receipe in the table below.
| Reagent | Volume |
|---|---|
| Lab taq | 0.5 |
| NEB Taq buffer | 10 |
| VRI 1 (fwd) primer | 2 |
| VRI 2 (rev) primer | 2 |
| dNTPs | 2 |
| npH20 | 13.5 |
Added 5 ul BglII digest sample to 25 ul PCR master mix and amplified for 34 cycles. Included 2 controls one being BglII digest without VR24 vector and second control with PCR MM only for a total of 3 PCR reactions.
11/30/21 #
VR16 KpnI digest gel #
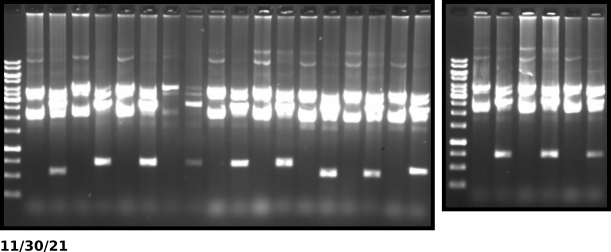
Gel layout #
All ladders are 1kb O’gene rulers. From right to left samples are ordered by ascending colony number (12 total samples). There are two lanes per sample with the right lane being the undigested control and the left being the KpnI digested sample.
VR24 fragment check #
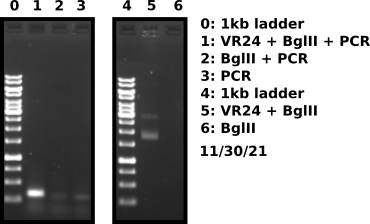
VR24 Gibson assembly #
| Reagent | Volume (ul) |
|---|---|
| VR24 PCR fragment | 0.5 ul |
| pFC9 LF 3 | 0.937 |
| Gibson master mix | 7.518 (11/21/21) |
| npH20 | 1.045 |
Made three total samples, 2 with 0.5 ul PCR product and one without to serve as a negative control. Incubated all samples at 50C for 1 hour in the thermocycler. Then transformed NEB DH10B cells with 5ul of each sample and used 100 pg of pUC as a positive control (lot 10119396).
12/1/21 #
Preparation of VR24 overnights for mini prep #
Using VR24 transformation colonies (see 11/29 notes) inoculated 24 2ml LB + Amp (100ug/ml) + 0.1% glucose with one VR24 colony using pipette tips. Placed into 37C room (third floor) with shaking at 200 rpm at 3:00 pm.
12/2/21 #
Where are the rest of the notes?
On 12/7/21 transistioned away from the one-day-one-file paradigm and replacing with one file per experiment. All previous T7 initiation series cloning notes are located in 7-20-21->10-12-21 folder which has notes on this topic starting on 7/20/21 till 10/12/21.
VR24 mini preps: lysis step #
Collected cultures from 37C and followed standard mini prep protocol (see GitHub page). Did protocol without incident. Stopped at LiCl freeze step and left in freezer overnight.
12/3/21 #
VR24 mini preps continued #
Completed LiCl purification #
Completed LiCl purification of all 24 samples. Re-suspended in 60 ul 10 mM Tris Hcl.
KpnI digest #
Digested mini prep samples with KpnI according to the table below.
| Reagent | Volume (ul) |
|---|---|
| Plasmid | 2 |
| 1.1 10x Buffer | 2 |
| npH20 | 16 |
Digested samples for 1 hour at 37C.