pFC8, 9 and pFC53Tac transformation day three #
Colony overnight growth #
Came into the lab around 8 am and found that the pFC8 and 53tac samples grew but the pFC9 sample did not.

Plated two new pFC9 colonies into separate 1L flasks with ~500 ml LB and 0.05 g amplicilin (100ug/ml).

Placed into incubation room at ~8:30 am to grow.

pFC8 and 53tac midi-prep #
Followed Qiagen midi-prep protocol. Used all Qiagen kit reagents for pFC8 and all reagents I made on Tuesday for pFC53tac. Did not run restriction digest yet (still need to select enzymes to use). Results from nanodrop are below.
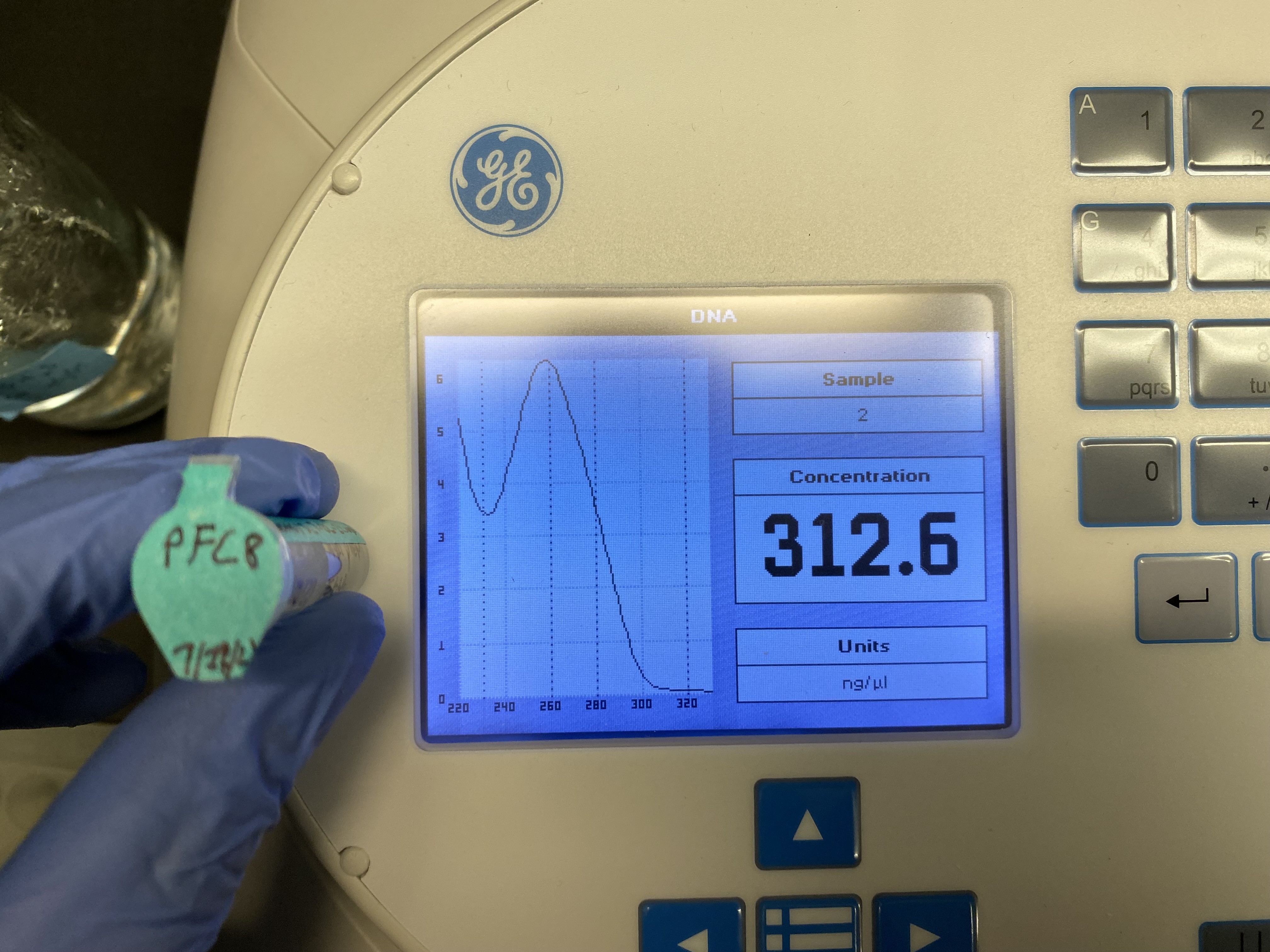
pFC8 nanodrop results #

pFC53tac nanodrop results #
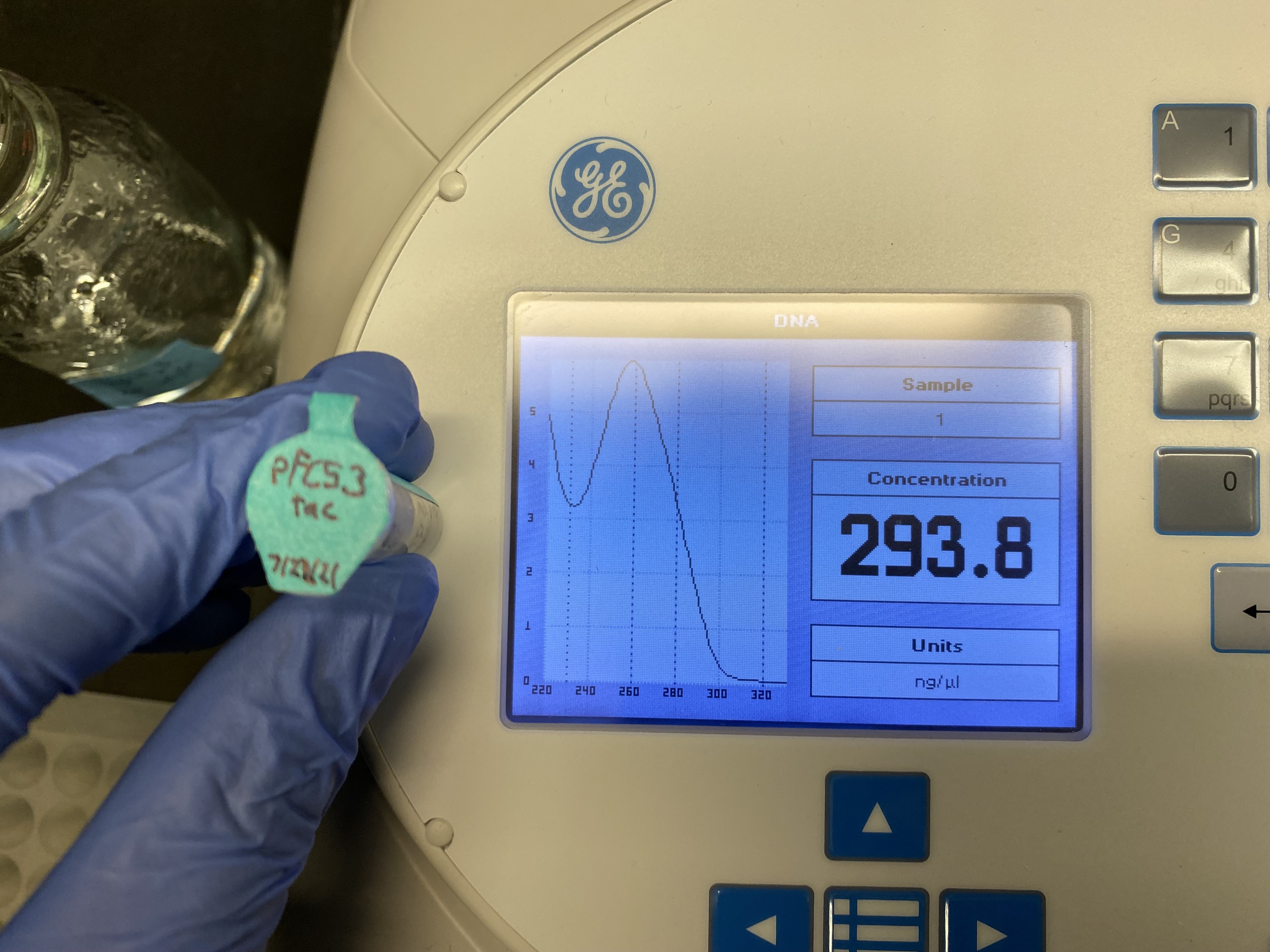

DNA concentrations #
| Plasmid | DNA (ng / ul) |
|---|---|
| pFC8 | 312 |
| pFC53tac | 293 |
Both samples were stored in -20 C kitchen freezer in the VR-inserts box.
Lessons learned #
This was a hell of a day. A combination of not really knowing what I was doing with this protocol and factors out of my control (centrifuge compatibility hell) lead to this prep taking just around 11 hours which is absolutely insane. So I have put together list of advice / tips for next midi-prep
- Cool all centrifuges to 4 C before starting anything
- Figure out what rotors you are using for each spin the day before
- SLA 1500 works in MCB centrifuge room with the 250 ml centrifuge flasks. These will generally be your best bet for larger volumes.
- The centrifuge that uses SLA 1500 takes about 45 mins to cool down to 4 C.
- The 50 ml centrifuge tubes with the weird caps will fit into the lab’s refrigerated centrifuge you just need to push down on the UFO rotor a bit when closing it.
- Minimize the number of separately treated sample tubes before running the columns. Running columns with large volumes will be a major time killer.