pFC9 EcoRI SacI digest #
In preparation for upcoming Gibson assembly reactions I needed to make more pFC9 large fragment. I digested pFC9 in 50ul volumes containing 1ug of plasmid. Below is 0.8 TAE gel of 5ul aliquots of each sample.
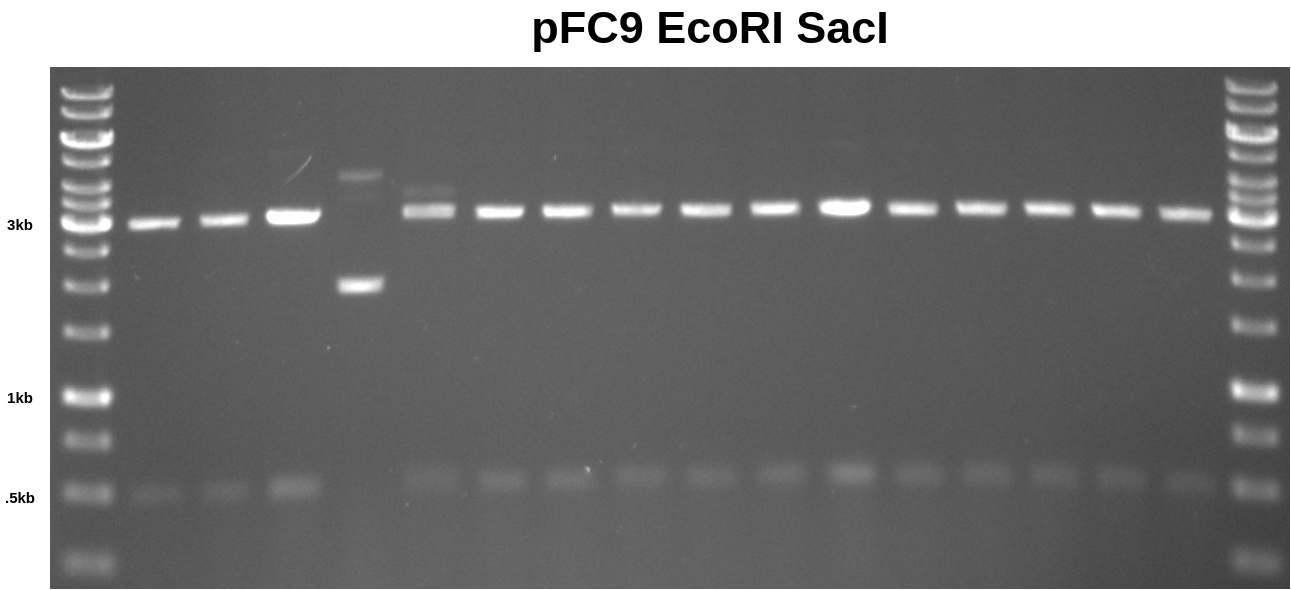
I discarded sample 4 (4th lane) and sample 5 (5th lane) due to the apparent under-digestion. I stored the remaining ~45ul of each sample in the VR inserts 2 box at -20C for later agarose gel purification of the large fragments.
Explanation of differences between PCR amplified vectors and inserts #
It turns out that the backbone used for pFC9 is the same plasmid that thermo used for cloning these inserts. This means that the homology arms used for Gibson assembly that target the backbone (5' homology arm) occur once in the insert and once in the thermo vector. This means that the forward primer for amplifying fragments actually has two binding sites for vectors but only one for fragments.
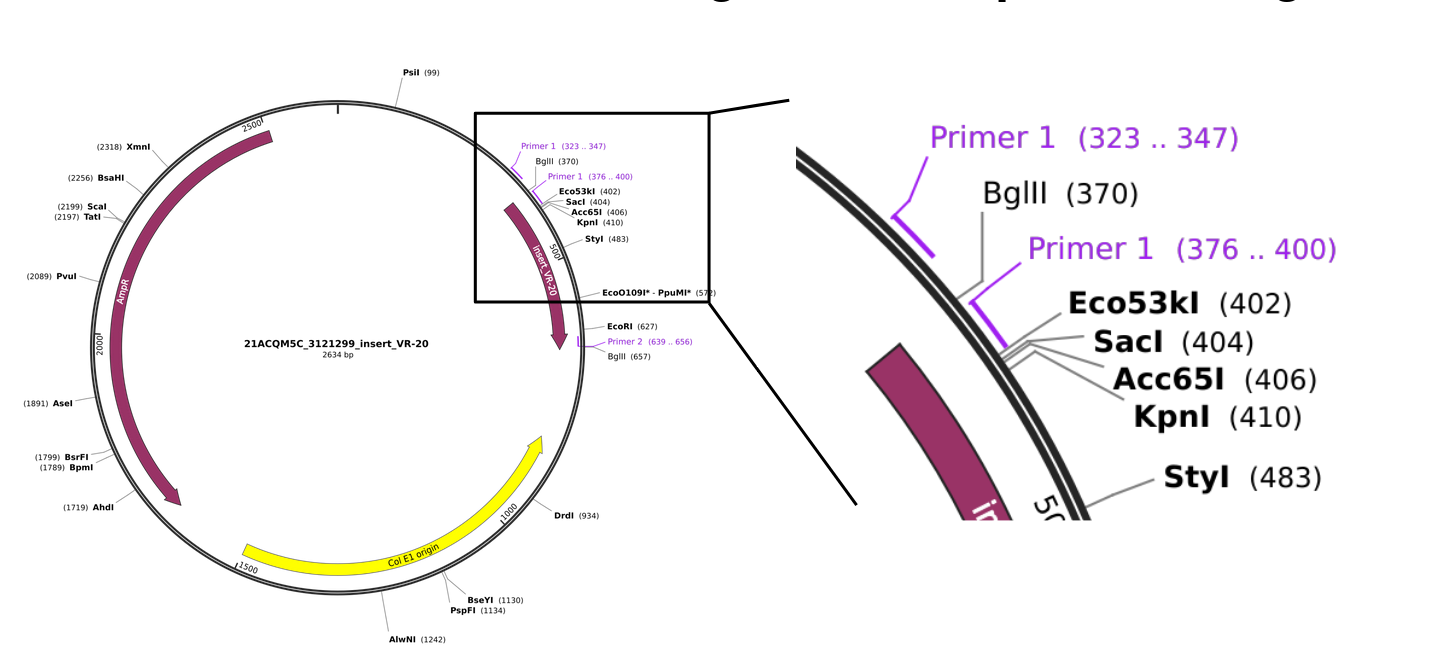
This results in vector amplified fragments being noticeably longer than fragment amplified vectors. This then requires digestion with BglII.