IVT practice with pFC8 and pFC11 #
IVT pFC8 #
DNA concentration: 740 ng / ul
| Name | Volume (ul) |
|---|---|
| 5x Buffer | 4 |
| 100 mM DTT | 4 |
| 2.5 mM NTP | 1 |
| DNA | 0.81 |
| H20 | 10.19 |
Used T7 Pol but was informed by Fred that pFC8 does not actually have a T7 promoter (oops) so this was never going to work out and is hopefully why the other gels failed as well.
Gel image #
No shift since not using the correct POL.
0.8 % TBE agarose ran 90v for 1 hr with 1ul 1kb Thermofisher O’Gene ruler.
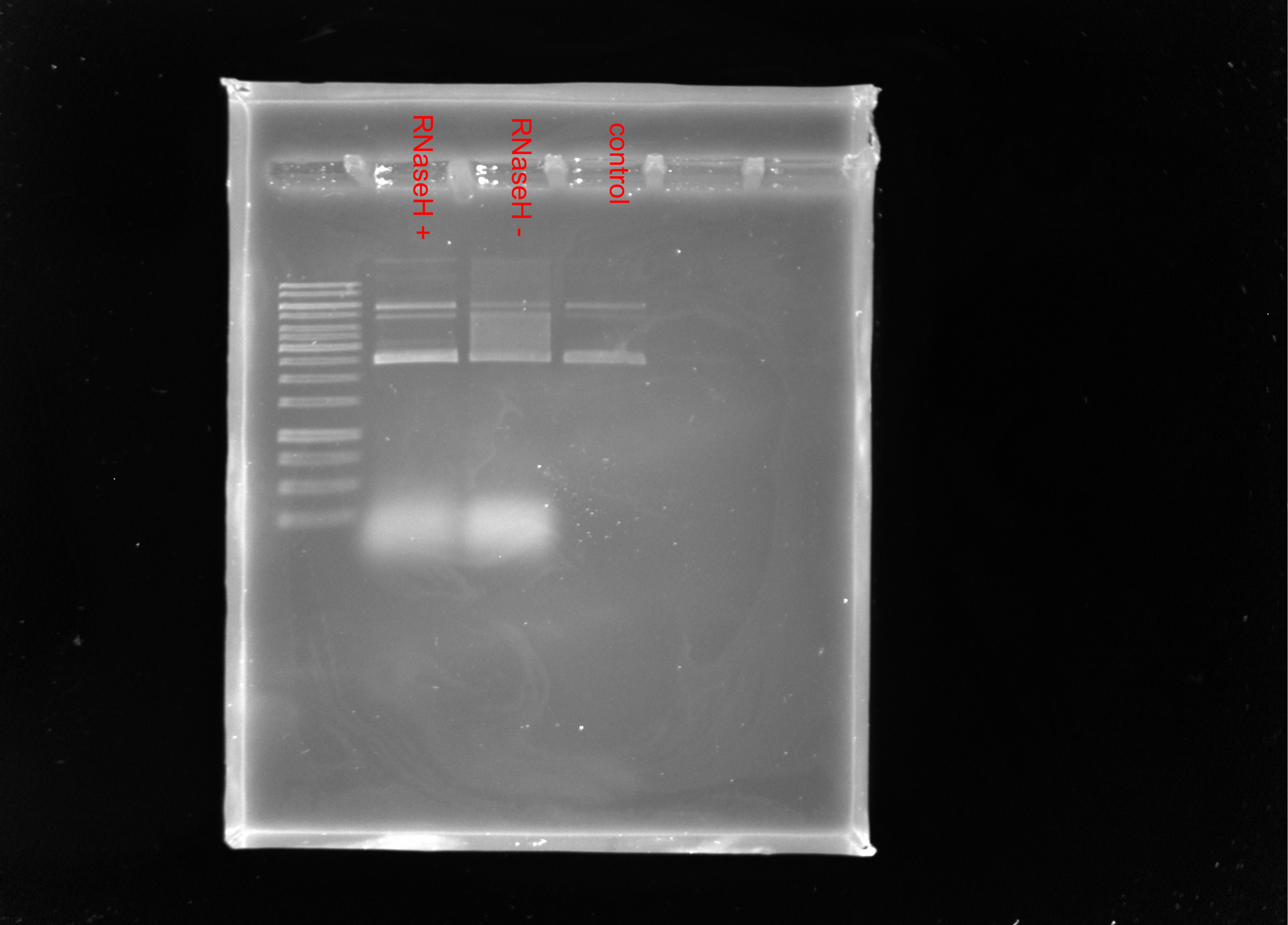
Redo pFC8T1T2 with T3 Pol + pFC11 with T7 Pol #
| Plasmid | DNA Concentration (ng / nl) |
|---|---|
| pFC8 | 240 |
| pFC11 | 252 |
Master mix reagents #
| Reagent | pFC8T1T2 | pFC11 |
|---|---|---|
| 5x Buffer | 4 | 4 |
| 100 mM DTT | 4 | 4 |
| 2.5 mM NTP | 1 | 1 |
| DNA | 2.5 | 2.38 |
| H20 | 8.5 | 8.61 |
Tube labeling #
Order in gel (right to left) is the same.

| Symbol | Meaning |
|---|---|
| + | RNaseH containing |
| - | RNaseH control |
| c | Un-transcribed |
\d | Plasmid number |
Gel image #
0.8 % TBE agarose ran 90v for 1.25 hr.
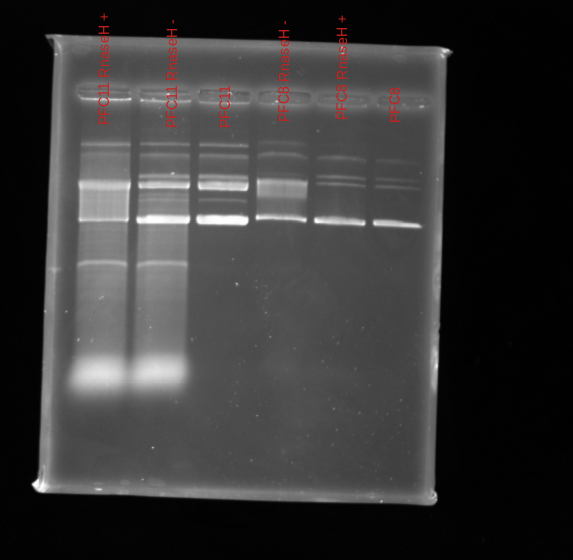
Looks like 11 was transcribed but no shift from the control. This actually makes sense because read through Fred’s plasmid doc and found that does not actually form R-loops. On the other hand pFC8 should form R-loops.
Maybe the T3 Pol is old and not good? Test with additional T3 plasmids and replicating pFC8.