Preparations #
11/30/21: Chemically competent cells #
Inoculated 1L LB in a 4 L autoclaved flask with 5 ml DH10B cells from liquid culture grown at 37C and started at 7:30 am this morning. Inoculated 1 L LB at 5:35 pm and grow overnight at 19C second floor innova shaker.
12/1/21: Chemically competent cells continued #
Came into lab at 7:50 am to start recording OD of cells started yesterday.
OD readings #
OD reading are made against sample taken from LB before inoculating with live culture.
| Time | OD |
|---|---|
| 07:50:00 AM | 0.18 |
| 08:50:00 AM | 0.28 |
| 09:50:00 AM | 0.27 |
| 10:32:00 AM | 0.3 |
| 11:16:00 AM | 0.34 |
| 11:48:00 AM | 0.38 |
| 12:20:00 PM | 0.43 |
Placed cells on ice. Followed Al-basam provided lab protocol and dispensed cells into 0.5 ml tubes in 200 ul aliquots. Daisy and Megan dropped tubes into LN2 as they were filled. Stored samples in -80C freezer.

Competency test #
After freezing thawed 4 aliquots of cells to test efficiency using NEB protocol for transformations. Table below describes samples. Plated 1/10th total transformed volume.
| Sample | Volume plasmid (ul) | DNA mass (ug) | Plasmid |
|---|---|---|---|
| 1 | 0.5 | 25 | pUC19 |
| 2 | 1 | 50 | pUC19 |
| 3 | 1 | 100 | pFC9 |
| 4 | 1 | 10 | pFC9 |
Incubated at 37C overnight.
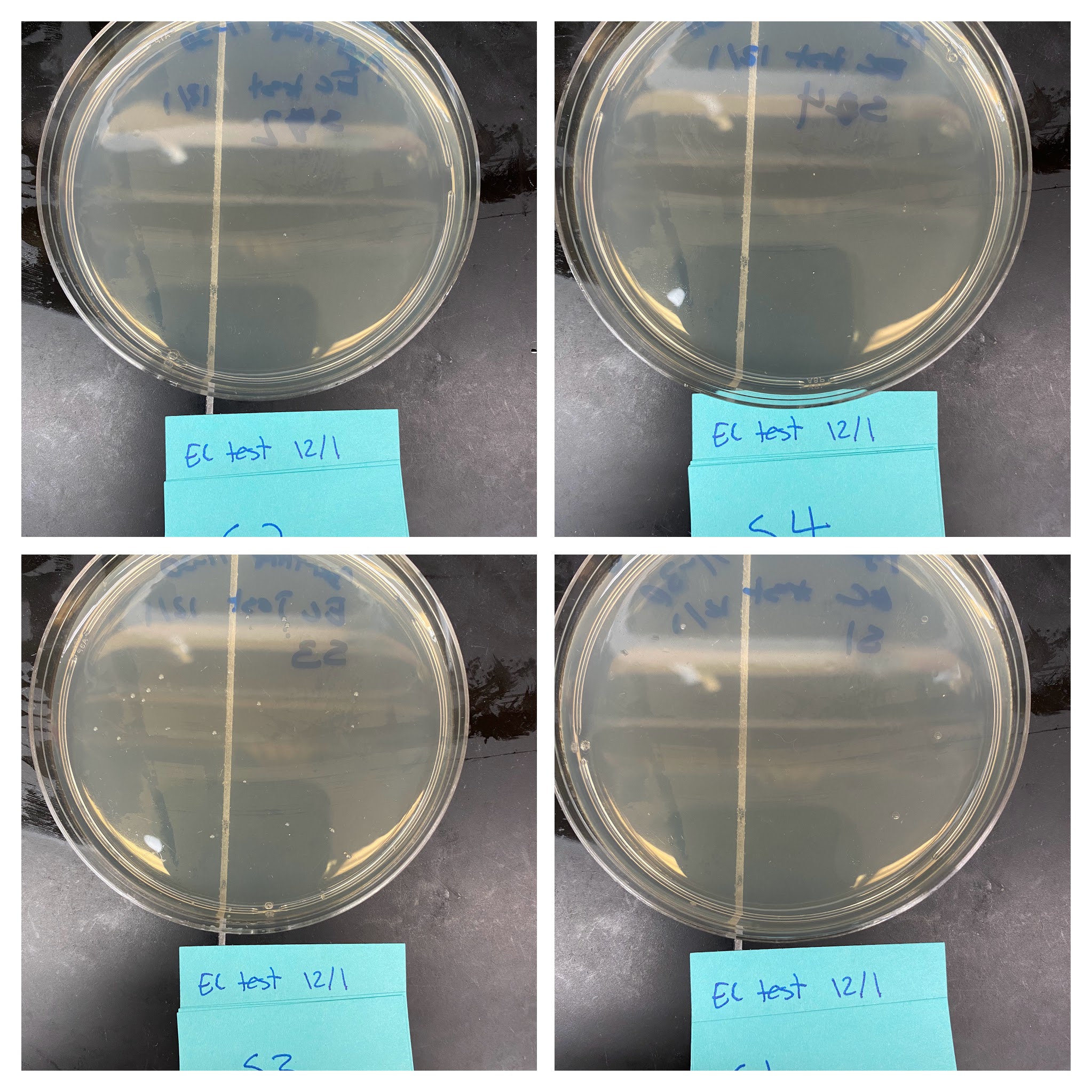
| Plate | DNA mass (pg) | Proportion plated | Number of Colonies | Efficiency |
|---|---|---|---|---|
| S1 | 25 | 0.1 | 2 | 8.00E+05 |
| S2 | 50 | 0.1 | 1 | 2.00E+05 |
| S3 | 100 | 0.1 | 20 | 2.00E+06 |
| S4 | 10 | 0.1 | 0 | 0.00E+00 |